J. Org. Chem.2021, 86, 5560−5567
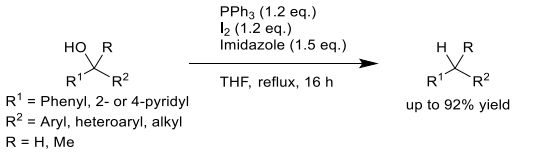
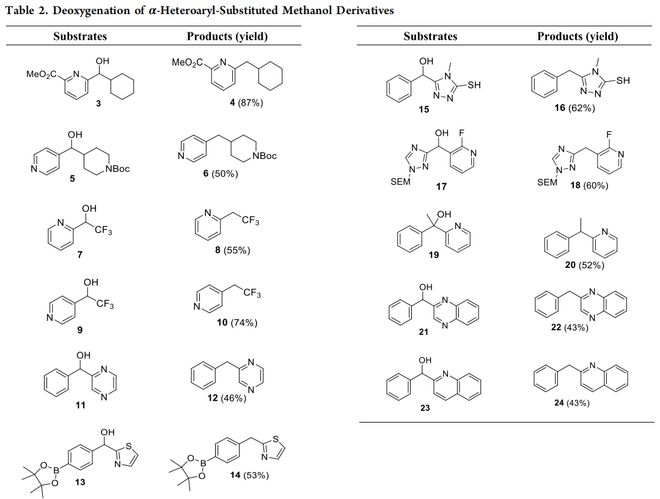
反应发生的关键,在于底物取代甲醇衍生物必须是含有一个或两个氮杂芳基取代的仲醇或叔醇,且杂原子与 α- 碳负离子处于共轭有效位置。

可以反应的杂环包含2 - 吡啶基、4 - 吡啶基,以及处于合适位点的喹啉,哌啶,噻唑,三唑,喹喔啉等。
不可参与反应的底物中,杂原子与 α- 碳负离子未处于共轭有效位置,如3 - 吡啶基取代的甲醇衍生物,处于3位的氮与 α- 碳的负电荷所在碳不处于共轭传递路径上,负电荷无法通过吡啶环共振到氮原子,无法稳定中间体,所以反应不能发生。
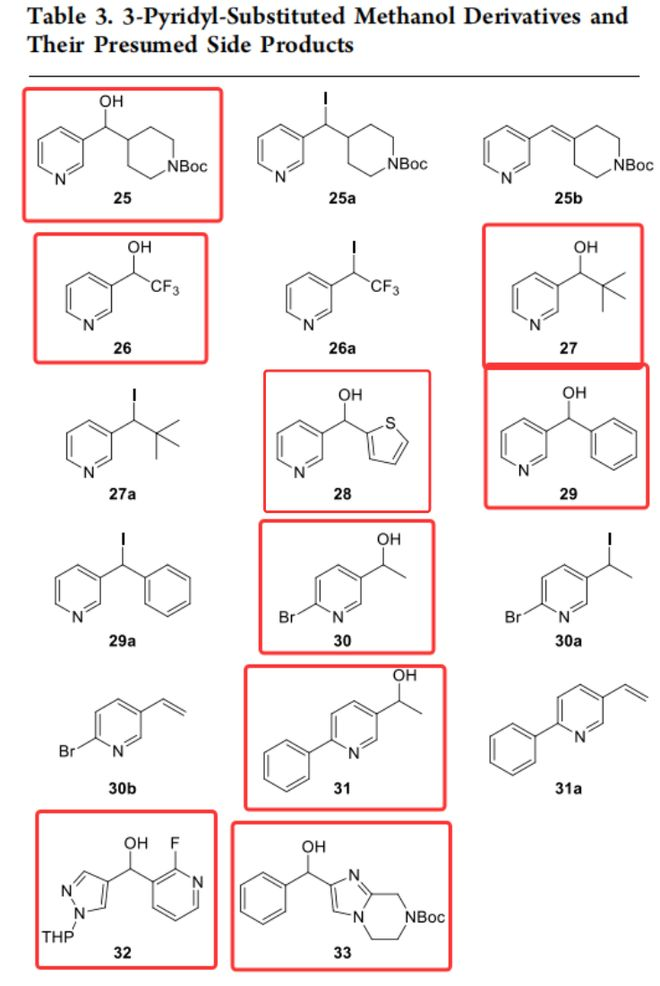
注:标红的为底物,未标红为这种体系中产生的副产物
通用反应流程如下:
General Procedure for the Synthesis of Deoxygenated α- Heteroaryl-Substituted Methanol Derivatives. To a mixture of α-heteroaryl-substituted methanol derivatives (1 equiv) in THF (2mL/100 mg) were added triphenylphosphine (1.2 equiv), imidazole(1.5 equiv), and I2 (1.2 equiv) at 25 °C under N2 protection. Then, the reaction mixture was stirred at the reflux temperature under N2 protection for 16 h. After cooling to room temperature, the mixture was diluted with water and extracted by EtOAc twice. The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue was purified by chromatography, Prep-TLC, or Prep-HPLC to afford the dehydroxyproduct.



















